TAU research discovers how “good” viruses can selectively destroy DNA of “bad” bacteria
Findings may assist in the development of treatments against antibiotic-resistant bacteria that cause infectious diseases
Support this researchA new study from Tel Aviv University (TAU) revealed a mechanism through which “good” viruses can attack the genetic systems of “bad” bacteria, destroying them and blocking their reproduction.
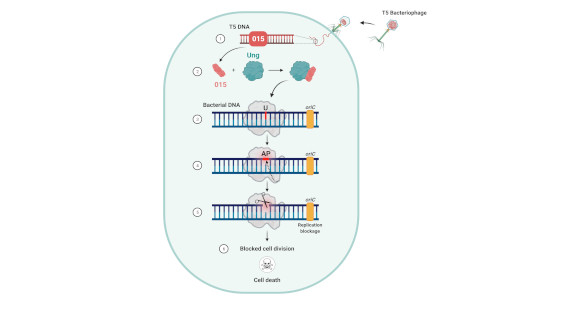
The researchers demonstrated that the “good” virus (bacteriophage) is able to block the replication mechanism of the bacteria’s DNA without damaging its own. Their discovery reveals one more aspect of the mutual relations between bacteria and bacteriophages and may lead to a better understanding of bacterial mechanisms for evading bacteriophages, as well as ways for using bacteriophages to combat bacteria.
The study was published recently in PNAS – Proceedings of the National Academy of Sciences and was led by Professor Udi Qimron, Dr. Dor Salomon, Dr. Tridib Mahata, and Shahar Molshanski-Mor of TAU’s Sackler Faculty of Medicine.
Professor Qimron explains that the antibiotic resistance of bacteria is one of the greatest challenges faced by scientists today. One potential solution may lie in further investigation of the targeted eradication of bacteria by “good” bacteriophages; namely, understanding bacteriophage mechanisms for taking over bacteria as a basis for the development of new tools to combat bacterial pathogens.
The current study unveiled the mechanism by which the bacteriophage takes control of the bacteria. The researchers found that a bacteriophage protein uses a DNA-repair protein in the bacteria to “cunningly” cut the bacteria’s DNA as it is being repaired. Since the bacteriophage’s own DNA has no need for this specific repair protein, it is protected from this nicking procedure. In this way the “good” bacteriophage does three important things: it distinguishes between its own DNA and that of the bacteria; it destroys the bacteria’s genetic material; and it blocks the bacteria’s propagation and cell division.
“The bacteriophage takes advantage of the bacterial DNA’s need for repair, while the bacteriophage itself has no need for this specific kind of repair,” Professor Qimron says. “In this way the bacteriophage destroys the bacteria without suffering any damage to itself. Its ability to distinguish between itself and others is of enormous importance in nature and in various biological applications. For example, all antibiotic mechanisms identify and neutralize bacteria only, with minimal effect on human cells. Another example is our immune system, which is geared toward maximum damage to foreign factors, with minimal self-injury.”
The researchers discovered the process by searching for types of bacterial variants not impacted by this bacteriophage mechanism – those that have developed “immunity” to it. This inquiry led them to the specific bacterial mechanisms affected by the bacteriophage takeover.
“We found that the ‘immune’ bacterial variants simply stopped repairing their DNA in ways that are vulnerable to the bacteriophage attack, thereby evading the bacteriophage’s destructive mechanism. Shedding more light on the ways in which bacteriophages attack bacteria, our findings may serve as a tool in the endless battle against antibiotic-resistant bacteria,” Professor Qimron concludes.
Other participants included Professor Tal Pupko, Head of TAU’s Shmunis School of Biomedicine and Cancer Research and its new AI and Data Science Center; Dr. Oren Avram of TAU’s George S. Wise Faculty of Life Sciences; and Dr. Ido Yosef, Dr. Moran Goren, Dr. Miriam Kohen-Manor, and Dr. Biswanath Jana of the Sackler Faculty of Medicine.